In 1980, the year I started my clinical studies, the World Health Organization announced that smallpox, one of the most dread infections in human history, had been eradicated. The new antibiotics and vaccines developed in the decades following the second world war had gone some way towards neutralising community fear of infectious diseases, and the success of the smallpox campaign reinforced the growing belief that the Age of Infections was coming to an end. From the 1960s on, Australian parents no longer had to face the terror of a polio epidemic; scarlet fever was rare, diphtheria was gone and tetanus going; even the diseases that were thought to be inevitable in childhood — measles, mumps and rubella — were disappearing. Cancer and heart disease were emerging as the big killers: forget about germs, it was our lifestyles that needed to change. This was, of course, a premature claim of Mission Accomplished.
New infectious diseases attenuated the medical triumphalism in the late 1960s and 1970s. An epidemic of arthritis and neurological symptoms associated with a distinctive rash occurred in a town called Lyme on the east coast of the United States; a number of young women, first in the United States but then in other countries, contracted a sometimes lethal condition known as toxic shock syndrome, found to be the result of a toxin produced by Staph aureus that contaminated their tampons; an epidemic of serious viral hepatitis was detected in recipients of blood transfusions and in countries where injecting drug use was becoming fashionable. But the diseases that concerned health authorities most — one in Europe and the others in Africa — were the newly recognised causes of viral haemorrhagic fever, or VHF.
VHFs can result from any of a number of different viruses with distinct structures, epidemiologies and means of transmission. They are linked together by the fact that they are all RNA viruses, they are usually confined to specific geographic areas, humans are not the natural reservoir and they are among the most deadly of all known infections. VHFs are characterised by severe, but usually non-specific, flu-like illness, followed in a proportion of cases by low blood pressure, which leads to organ failure (“septic shock”) and, in the most severe cases, bleeding into the conjunctivae, skin, mucous membranes or bowel. Although bleeding is one of the feared parts of VHFs, it is a sign that the patient is critically ill rather than a cause of death itself.
VHFs are not new. One of them, yellow fever, has been known as a human disease for hundreds of years. It came to prominence in the early twentieth century when the American physician Walter Reed proved that the great killer of the workers on the Panama Canal was transmitted by mosquitoes, the first time an insect had been unequivocally found to be a “vector” in the transmission of an infectious disease. The viruses responsible for other VHFs were successively identified from the 1960s on. Crimean-Congo haemorrhagic fever, for instance, is caused by a tick-borne virus. Dengue fever, an increasingly common mosquito-borne infection in many parts of the world, including northern Australia, can act like a VHF if a previously infected patient is exposed to a different dengue subtype. Other VHFs include Lassa fever, Hantavirus and Rift Valley fever.
If the VHF is insect-borne, then there is no risk of transmission in places that are free of that insect. But what if the infection, once it moves from its animal reservoir, can spread from person to person?
In 1967, hospitals in the German cities of Marburg and Frankfurt admitted a number of very sick patients with fever, severe flu-like illness, conjunctivitis, nausea, vomiting and diarrhoea. A handful of patients with similar symptoms were also seen in Belgrade. The patients were originally thought to have a Salmonella or Shigella infection of the bowel, but the usual stool and blood cultures were all negative. About a quarter developed a severe derangement of their blood coagulation and bled from the mouth, lungs, bowel and needle-puncture sites. In all, thirty-two patients were seen and seven died.
Each of the Marburg patients was associated in some way with the pharmaceutical company Behringwerke; those in Frankfurt had some relationship with the Paul Ehrlich Institute; and one of the Belgrade patients was a veterinarian researcher. Within just three months German virologists identified the first of the human filoviruses to be discovered and named it Marburg virus.
The likely source of infection was infected cells, blood and other tissues derived from a consignment of Cercopithecus aethiops monkeys that had been imported from Uganda in June 1967, right in the middle of the Israeli Six-Day War. Because of the conflict, the monkeys were delayed in transit in Britain and then by a strike at Heathrow Airport. How or when the monkeys became infected with Marburg virus has never been established but it is known that they came into close contact with a shipment of South American finches and langur monkeys from Sri Lanka in an animal house near the airport. Two of the monkeys escaped into London but apparently didn’t transmit any disease there.
No further cases of illness associated with Marburg virus were identified in Europe. The next case came in 1975 when an Australian backpacker died in South Africa after falling ill while travelling in Zimbabwe. His female companion and a nurse at the South African hospital where he was treated developed a milder illness. Both recovered and were found to have antibodies to Marburg virus. In 1990 a Russian laboratory worker was fatally infected. Only two other substantial epidemics of Marburg virus have been recorded: an outbreak in the Democratic Republic of the Congo in 1998, which killed 118 of the 154 people affected, and 227 people died among 252 identified cases in Angola in 2004.
The sudden appearance and disappearance of Marburg virus in Europe was puzzling. Only those who had been in direct contact with infected tissues and other samples in the laboratories seemed to be at risk, and only a very small proportion of those who cared directly for those with symptoms became ill themselves. Subsequent blood testing showed very few, if any, people without symptoms developed antibodies to Marburg (known as “asymptomatic” infection). Viruses such as influenza, chicken pox and measles can be spread through the air in droplets and aerosols, but the pattern of spread seen in the Marburg outbreak made it unlikely that airborne transmission occurred. In fact, it seemed to be quite hard to become infected with the virus — but if you did, you had an extremely high chance of dying.
New VHFs continued to appear. In 1969, two nurses died of a VHF-like illness contracted in a town named Lassa in Nigeria. A third nurse looking after them in a mission hospital became critically ill and was repatriated to New York. Her blood was sent to a Yale University laboratory where the head, Jordi Casals, and a research technician contracted the illness. The technician died but Casals recovered and went on to isolate a new arenavirus, which was named Lassa. The disease was subsequently found to be endemic in West Africa and the World Health Organization estimates that there are between 300,000 and 500,000 Lassa fever cases each year.
In 1976 a Belgian doctor working in the Democratic Republic of the Congo (then Zaire) sent the blood of a dying nun to the Institute of Tropical Medicine in Antwerp, hoping to identify the cause of her illness. Standards for the transport of biological materials were different then, and by the time the thermos flask arrived in Belgium the blood had leaked out of its tube into the defrosted ice water that was keeping the sample cool. Despite the poor state of the sample, electron microscopy immediately identified the presence of a filovirus that looked very similar to Marburg virus but was subsequently shown to be a distinct viral species. Although an epidemic appeared simultaneously in the Sudan, the virus was named Ebola after the river that ran through one of the main areas affected in the Democratic Republic of the Congo. It soon became apparent that the Ebola virus was one of the most deadly human viral infections — in Africa the case-fatality rate appeared to be as high as 90 per cent.
It is worth remembering that emerging diseases often record very high mortality rates. Only the most severe cases are seen in the early stages and, not surprisingly, they have the worst outcomes. Indeed, many diseases that appear to have very severe clinical manifestations are often mild or even asymptomatic in a majority of cases. The Spanish influenza pandemic of 1918, considered to be the worst influenza pandemic in history, had a case-fatality rate of around 2 to 3 per cent. Its notoriety results from the interaction of that rate with an “attack rate” — the proportion of the entire population that is infected — of at least 30 per cent. Three per cent of nearly a third of the entire population translated into millions of deaths globally. The 90 per cent mortality among several hundred early cases of Ebola obviously had a different absolute effect on the population.
The rapid growth in international travel in the 1970s meant that the jumbo became as important a vector for the spread of disease as the mosquito. The medical profession began working out how to minimise the risk of an outbreak of VHF in the developed world, and one of the results was that millions of dollars were invested in hospitals and laboratories across the world to deal with these viruses.
In 1982, the Fairfield Infectious Diseases Hospital in Melbourne was the last of the Australian “fever hospitals.” No other state had kept their equivalent quarantine hospitals open, and the care of infectious diseases had been devolved into the mainstream health system. So this was the logical site for a high-security ward to house patients with suspected VHF, and for a Level 4 biocontainment laboratory where blood samples could be tested and the viruses grown.
I toured the newly commissioned facility when I was doing my two weeks at Fairfield as a medical student. The austere brick structure with tiny windows and exhaust vents protruding through the roof was about one hundred metres away from the rest of the hospital. It looked safe. When in operation, the inside of the building was kept at a slightly lower atmospheric pressure so that air from the outside moved in, rather than the other way round, to reduce the risk of an escape of any infectious material.
One of us was allowed to get dressed up in the positive-pressure biosecurity suit. Now this was just like being in a movie (for us it was The Andromeda Strain, based on the Michael Crichton book from 1969; Outbreak, with Dustin Hoffman, was still more than a decade in the future). The suit was hot, stuffy and claustrophobic and the student who volunteered for the demonstration looked slightly dehydrated and a little worse for wear when she emerged after half an hour.
I remember being very impressed with the whole set-up. But when I returned a few years later as a junior doctor I learnt that the unit had been the baby of a senior doctor who was now in a different sort of quarantine — professional in this instance. Although the reasons for his exile were never spoken out loud, there was clearly great antipathy between him and the rest of the staff, who usually referred to the facility as the White Elephant. One of the doctors was pleased to tell me that the first time the negative pressure system was switched on part of the ceiling collapsed. Not that there was much risk of hurting any patients; the unit had only ever housed one, an African man with a fever who was transferred from another state.
The plan was for a patient with a suspected VHF to be transferred from his or her place of diagnosis to Fairfield in one of ten portable high-security quarantine “cots” that had been built by the federal government. The African patient was brought back to Melbourne by the retrieval team and this attracted a considerable degree of media attention. He was admitted to the negative pressure ward, the ceiling of which remained in place this time, treated by the doctors and nurses in their sci-fi pressure suits, and very soon diagnosed with Streptococcal pharyngitis. He was reported to have been very impressed with the Australian medical system and the way it cared for people with a sore throat.
In 1989 I took part in what was to be the last training flight of the retrieval team. We flew to Cairns on an Ansett 707, off-loaded the cot and drove to the Base Hospital to pick up our pretend patient. One of our nurses volunteered to spend the return trip in the plastic-encased, negative-pressure cot. We practised caring for the patient in mid-air but no one seemed to be taking things too seriously — by then the medical world had learnt that the viruses that caused Lassa fever and Ebola were not transmitted through the air and could be easily contained with standard precautions to avoid direct contact with blood and other bodily fluids.
One other thing had changed. For the past eight years staff at Fairfield had treated hundreds of patients with a then universally fatal virus in their blood, HIV. We had been right to be worried about the movement of viral diseases in and out of Africa — we just became preoccupied with the wrong virus. While the medical profession had been working out how to minimise the risk of an outbreak of VHF in the developed world, an African virus just as deadly but much more insidious in its action was quietly establishing itself across the globe. HIV would soon become the worst pandemic of the twentieth century and it would push fears of Ebola, Lassa and Marburg into the background.
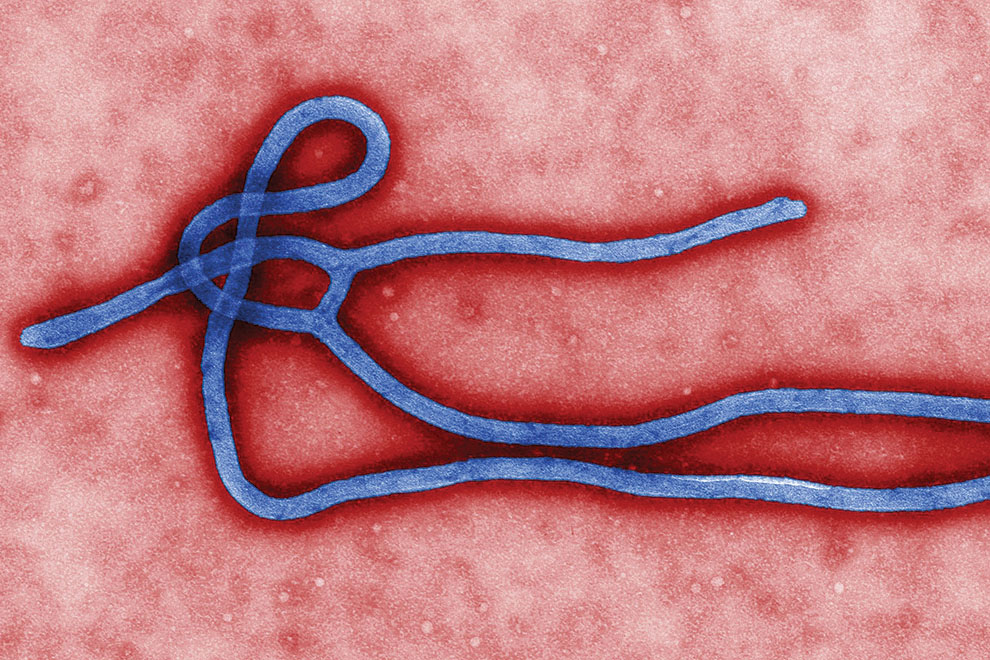
Under an electron microscope, the slender, filamentous appearance of Ebola looks like an Egyptian hieroglyph. It contains a remarkably simple, and scary, piece of ribonucleic acid that encodes for fewer than ten proteins that are manufactured by virus-infected human cells. These proteins pack a very powerful punch: they stimulate the release of chemicals, known as cytokines, into the local tissues and into the bloodstream, which in turn produce a profound inflammatory reaction. One of the proteins directly damages the cells that line the blood vessels, which results in the bleeding typical of the more serious cases. It is the intensity of the immune response that makes the patient look and feel so sick. As is the case in many fatal infections, the immune response is maladaptive and, rather than helping patients recover, it actually contributes to their demise.
Unlike Marburg, which only has one viral strain, there are five known subspecies of Ebola virus. One of these, the Reston strain, caused considerable concern in 1989 when it was identified in monkeys held in a laboratory in Reston, Virginia, near Washington DC. Three laboratory workers, including one who sustained a needlestick injury from one of the infected animals, developed antibodies to the virus but did not become ill, suggesting that the risk of human disease is low.
Ebola is probably thousands of years old and has been quietly reproducing in a non-human reservoir for most of that time. The best evidence at the moment is that bats are the natural reservoir. It is only when the virus is transmitted to humans and other primates that it causes symptoms of disease. Transmission is thought to occur when humans kill, prepare or eat infected bats and bush meats (including monkeys). The occasional explosive outbreaks of Ebola in humans are really just an accidental sideshow to the main cycle of transmission.
That didn’t stop the feeling of panic in March this year when the World Health Organization revealed that an Ebola epidemic had broken out in the west African country Guinea. There had already been at least twenty significant outbreaks of Ebola in Africa, with a total of 3000 reported infections. The case-fatality rate has ranged from around 50 per cent to 90 per cent, depending on the size and place of the outbreak. The current epidemic has spread to Liberia, Sierra Leone and Nigeria and is the largest ever recorded: as of late August there had been 2473 cases and 1350 deaths, giving a mortality rate of 55 per cent. The actual number of infected people is likely to be higher than this, owing to the limited local resources for case counting.
On 7 August this year the World Health Organization declared the Ebola epidemic a Public Health Emergency of International Concern. The key word here is “international,” because it is hard to argue that it is a crisis in African terms, given that the continent is subject to so many other infectious diseases. This epidemic will need to be at least eighty times worse if it is to kill as many people as yellow fever did in 2013, or 500 times worse to reach the annual death toll for malaria in Africa. Lassa fever is estimated to kill at least 5000 people each year.
But diseases like Ebola draw attention to themselves in a way that other viruses don’t. The fact that at least one-in-two people with Ebola die within days of becoming ill gives the disease a Hollywood cachet, but the fact that it is so obviously an infectious disease is what seals its ability to cause fear and loathing.
Throughout history there have been diseases that are very clearly infectious — cholera, the Black Death and influenza, for example. With these diseases the time between exposure to a patient with the illness and onset of symptoms in the exposed is so short that it is easy to see the association, even if the exact nature of the contagious material is not as obvious. It takes a much more sophisticated analysis to work out the cause of diseases with longer incubation periods or where asymptomatic infection is common.
Tuberculosis, today a paradigmatic example of an infectious disease, was thought to be an inherited condition until the nineteenth century. Hepatitis B has been present in human populations for tens of thousands of years but because it rarely causes symptoms in newborns and children, the ages when most transmission happens, its existence was not even suspected until 1885 when an outbreak of jaundice occurred in Germany in the recipients of contaminated smallpox vaccinations. (The first epidemic of Ebola was also probably fuelled by contaminated injections, given by the very nun whose blood provided the evidence of the virus’s existence.)
The developed world is fearful of the effect that Ebola will have outside Africa and, ironically, it will be the West’s response to this threat that will control the disease at its source. Ebola epidemics occur because of fundamental weaknesses in health infrastructure in Africa, not because of a lack of expensive (or even cheap) drugs and intensive care units. Ebola persists because of the health system’s inability to provide adequate protective equipment such as gowns, goggles, masks and gloves. The safe containment and interment of the bodies of those who have died is also a major issue in any setting of mass death — but never more so than when the dead pose a true threat to the living.
There is no drug on the market that has been shown to be effective for the treatment of any of the VHFs. An experimental formulation containing a mixture of antibodies has been used on a handful of patients with Ebola but there have been no formal trials of its efficacy.
The current epidemic has been a spur to vaccine research and there are a number of candidates ready to enter Phase 1 trials. Despite some encouraging results in animals, there is no guarantee that the vaccines will work in humans, and it is possible they may be not just ineffective but also harmful, as has been seen with some other vaccines. Testing the real-world value of such a vaccine is also difficult because of the sporadic nature of the epidemics.
Regardless of its efficacy, the funding for the roll-out of an Ebola vaccine is not guaranteed: a safe, cheap and effective yellow fever vaccine has been available for nearly eighty years but vaccination coverage in Africa was virtually zero until a decade ago. The GAVI Alliance, backed by Bill and Melinda Gates, is working to increase the rate of vaccination but the coverage in Africa is not yet sufficient to interrupt transmission.
Earth has never had so many humans living on it. We travel obsessively, we progressively encroach on jungles and other remote areas, we interact with domestic birds and animals in new ways, and we eat meats that were never part of human diets in the past. It is inevitable that viruses that have been quietly reproducing in animals, causing little harm, will move into human populations and cause much harm. Inevitable but unpredictable.
It is impossible to know how the current Ebola epidemic will unfold, but it is likely to go the way of all previous outbreaks once strict compliance with infection control has been achieved. All viruses evolve in response to changes in their environment, though, so it is possible that the strain responsible for the 2014 outbreak has mutated. Since it is already one of the deadliest viruses known to science, the most worrying change would be one that makes the virus easier to transmit. It is unlikely to go through the significant, and unprecedented, genetic alteration necessary to spread via aerosols or droplets. But if a change of that nature ever did happen then that would constitute a Public Health Emergency of International Concern. •